Table of contents
Generally, PU are obtained by the polyaddition reaction between a diol and a diisocyanate, therefore are highly petroleum dependent. Moreover, PU residues has increase severity, hence the concern about its disposal. In other words, because polyurethanes are petrochemical-based polymers, it is important that we recycle them whenever possible, so that precious raw materials do not go to waste.
At first, people directly dealing with polyurethane wastes is landfill or burning. Because of small polyurethane foam plastic pile-up density, landfill will be serious waste of land resources. At the same time polyurethane wastes are very difficult to decomposition in the natural conditions.
There are various recycling options, including mechanical and chemical recycling. Depending on the type of polyurethane, different ways of recycling can be applied.
Attempts to recycle polyurethane by chemical reaction are nearly as old as this class of polymers. First, polyurethanes were produced as fibers in competition to the polyamides, which were developed in the late 1920s. the development of polyurethanes, thus, started in the 30s with the aim to produce fibers, and elastomers ad rigid foams followed in the early 40s. already in the late 1940s scientists in Germany and in USA thought about reversing the reaction of formation of polyurethanes to produce again the polyol and the polyisocyanate as it was found that the temperature of decomposition is generally in the range of 200. After some 20 years of little activity new investigations on the recycling of polyurethanes started in the mid-1960s. In this time the major path to decompose polyurethanes was the hydrolysis with steam without or with pressure and temperatures up to 450. Today, there exist for polyurethanes probably more technically feasible recycling processes than for any other class of polymers.
It has to be mentioned that as the main products in the class of polyurethanes are foams, focus of the recycling processes was on these materials.
Ways to recycle polyurethanes
- Thermal methods
Thermal methods maybe subdivided into the three categories: pyrolysis, incineration of wastes, and thermal cleavage to receive defined compounds.
Pyrolysis was attempted several times to produce useful materials such as synthesis gas or small unsaturated compounds to be used in radical polymerization. Since this type of recycling is not of any industrial importance at presence due to non-specific reactions and products it is not considered further.
Incineration was attempted with single polyurethane products to give heat and electrical energy and as a high energetic addition fuel to waste incineration plants. Polyurethanes contain a considerable amount of thermal energy in the range of 30 MJ/kg. Several years it was feared that in incineration polyurethanes lead to either cyanides or hydrocyanic acid. Both have been found in significant amounts. But the only noxious compound to be considered is carbon monoxide which is produced from the aromatics in significant amounts and has to be eliminated from the exhaust gases by afterburning.
Incineration as a way in the recycling of polyurethane wastes occupies an important position, especially for those who can’t use other methods of recycling wastes, burning can be a kind of good method. But if the Incineration process is incomplete combustion, it will produce poisonous gas which seriously polluted the atmospheric. Therefor the method is being phased out gradually.
Thermal cleavage has been tried to produce isocyanates on this way. Therefore, special oligo- and polyurethanes have been designed to produce high yields of isocyanates. According to recent information the processes developed so far produce yields in the range of 25 to 27% b. w. of aromatic isocyanates and somewhat higher yields with aliphatic diisocyanates. The increase in isocyanate prices may lead to a re-evaluation of this process to use the easier to obtain aromatic di- or polycarbamates and to avoid use of phosgene in the typical way to produce the isocyanates as it is common today.
Hydrolysis of polyurethanes
The hydrolysis of polyurethanes, i.e., the reaction with water or steam under elevated conditions, was the first type of recycling used by the Ford Motor Co. in the sixties. The process is depicted below:
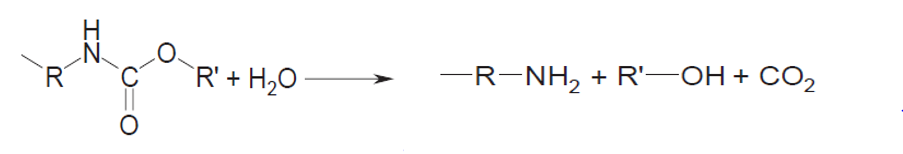
The conversion was performed at temperatures of about 300 and pressures of about 15 atm but further development of the process resulted in somewhat more extreme conditions, i.e. temperatures above 450 at a pressure in the range of 50 atm. The energy consumption is further increased by the necessity to completely separate the amines from the polyether polyols. This complete separation is necessary to be able to use the amines thus produced in the phosgenation reaction to produce new isocyanates from them.
It can be said that hydrolysis is but a side reaction in other ways to chemically recycle polyurethanes since any polyurethane foam contains more or less absorbed water. On the other hand, water will react in chemical recycling processes always in side reactions giving undesired products, especially primary aromatic diamines such as 4,4-diaminodiphenylmethane (MDA) or 2,4- and 2,6-toluene diamines. These are not only cancerogenic substances but also affect the quality of the recycling polyols in another way; they increase their viscosity due to thixotropy.
Alcoholysis of polyurethanes
The formal substitution of one hydrogen atom in water by an aliphatic group leads to alcohols which may as well be used as cleavage reagents for polyurethanes. The first alcohol to be used was methanol. The reaction was performed in a similar way as the hydrolysis at high temperature under pressure. It was hoped to more easily separate the amines from the polyol methanol mixture. It turned out that the separation was nearly as difficult as with the hydrolysis products but additionally methanol had to be evaporated. Higher alcohols such as butanols were used as well but having no better results.
Glycolysis of polyurethanes
Glycols are divalent alcohols with higher boiling point so that at elevated temperatures no pressure has to be applied. The glycolysis has another advantage: since the glycols react with diisocyanates to form leaner macromolecules as well they may be let in the reaction mixture as a component to produce polyurethanes. Glycolysis is performed as a single stage process with or without catalysts.
The glycolysis is usually performed at temperatures between 200 and 270 within three to ten hours. Catalysts do not significantly reduce the reaction times. Thus, three general types of glycolysis are to be seen:
- Non-catalyzed glycolysis by low molecular weight glycols such as diethylene glycol or propylene glycol or mixtures thereof.
- The catalyzed glycolysis in a similar way but with the addition of some types of catalysts.
- The glycolysis by the use of polyether polyols with or without adding catalysts. The lower reactive hydroxyl groups of the polyether polyols lead to longer reaction times so that the rate of reaction of this process was tried to be accelerated by the use of both catalysts and/or glycols.
Mechanical Recycling
Physical recycling method is crushing polyurethane foam wastes, only changing physical form. The smashing solid particles have no reactive activity, but directly make new polyurethane products as recovery processing of raw materials. Through mixed with adhesives, they can make all kinds of mold products by the compression molding method. This is currently the most widely used method.
Hot compression molding is one of the processes that do not require binders. Finely ground particles of polyurethane foam are condensed under high pressure and temperature (180 and350 bar). This technology is mainly used in the processing of rigid polyurethane foam waste from the automotive industry.
Mechanical Recycling Rebounded Flexible Foam: It is made by mixing pieces of chopped flexible PU foam and a binder, and it is used in carpet underlay, sports mats, cushioning, and similar products. Regrind or Powdering: PU industrial trim or post-consumer parts are grounded up resulting into a fine powder. The powder obtained is mixed with virgin materials to create new PU foam or RIM parts.
Physical recycling method is simple and convenient, with low cost, but there are still certain technical limitations at various physical recycling method processing. Performance of recovery products is poor, which only apply to some of the cheap products, and limit the market.

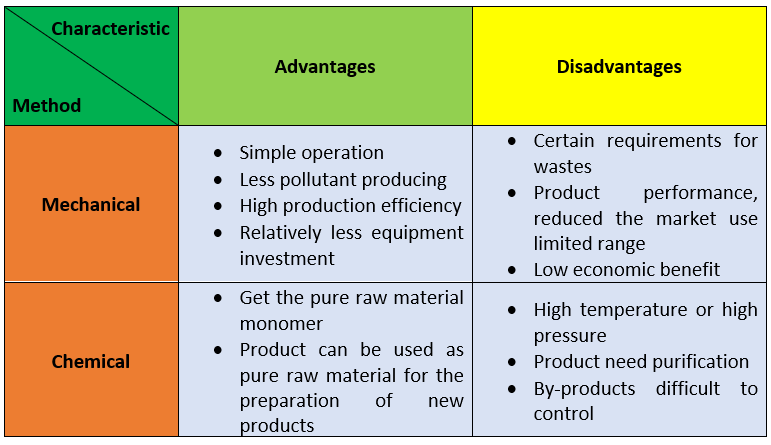
To sum up, there are two ways, physical recycling and chemical recycling, for recycling polyurethane foam wastes. They are compered in the following table: