Table of contents
The basic chemistry of flexible polyurethane foams is not difficult to grasp. It is the reaction of an alcohol or OH group with an isocyanate or NCO group. The alcohol is normally polyfunctional, ranging from 2–8 OH groups, which are referred to as polyols. As the number of OH groups increases, the foam structure becomes more rigid. Polyols are classified as polyether or polyester, based on the starter (initiator) materials used in their manufacture. The initiator is reacted with propylene oxide, ethylene oxide, or a combination of the two. The choice of which initiator and oxide to use depends on the foam characteristics desired. A variety of polyols are available to tailor the foam characteristics. The usual isocyanates for foam are toluene diisocyanate (TDI) and diphenylmethane diisocyanate (MDI). Various forms are available, again tailored to specific applications.
The foaming operation is complex because three basic reactions are occurring concurrently and at different rates. These reactions are chain extension, gas formation, and cross-linking.
CHAIN EXTENSION
The primary reaction is that of the isocyanate group of the alcohol to give a urethane linkage:
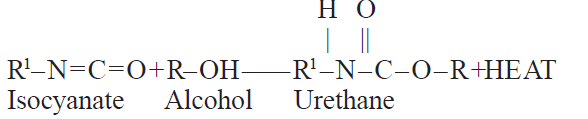
The urethane further reacts with additional isocyanate to yield an allophanate:
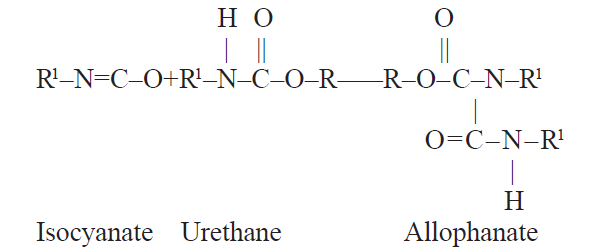
Isocyanate Urethane Allophanate The primary catalysts used for this reaction are organotin compounds; however, in very reactive systems, no catalyst may be required.
GAS FORMATION
Gas formation involves the reaction of the isocyanate with water to form an aromatic amine compound plus carbon dioxide in a twostep reaction. This carbon dioxide causes the cell formation and foaming. Until recently, the use of water in formulations for rigid foam other than in minor quantities was a major differentiating factor between flexible and rigid foams. A class of flexible foams known as integral skin foams was also formulated without water. Rigid and flexible integral skin foams were produced by adding auxiliary blowing agents to the reacting mixture. Initially, the primary blowing agents were chlorofluorocarbons (CFCs), specifically CFC-11 and CFC-12. CFC-11, or methylene chloride, was also used in flexible foams to manufacture lower-density and/or softer foams than were obtainable with water blowing alone. With the discovery that chlorinated compounds, such as CFCs and methylene chloride, destroyed the earth’s ozone layer, alternative materials were developed. These include completely chlorine-free hydrofluorocarbon compounds (HFCs), cyclopentane, and acetone. The alternatives have required modifications in processing equipment so they are used safely and efficiently. New formulation techniques also allow production of both rigid and flexible integral skin foams with water as the only blowing agent.
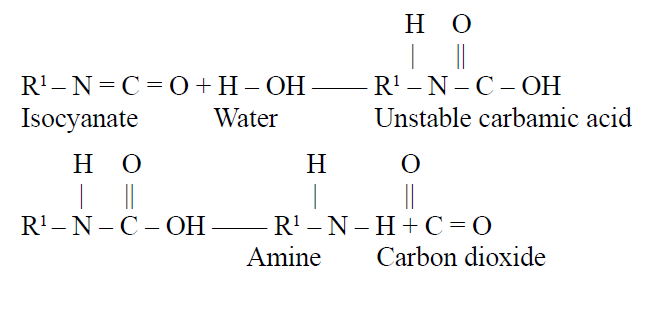
The catalysts for this reaction are primarily tertiary amines; however, some metal oxides have also been found effective. To stabilize the foaming mixture, silicone or other specialty surfactants are used.
CROSS-LINKING
The amine that is generated in the gas formation reaction reacts with more isocyanate to form a disubstituted urea, which cross-links the urethane polymer:
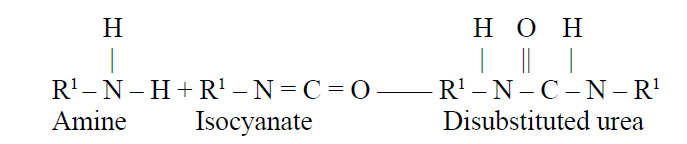
Some of the disubstituted urea then reacts further with isocyanate to form highly cross-linked biuret structures:
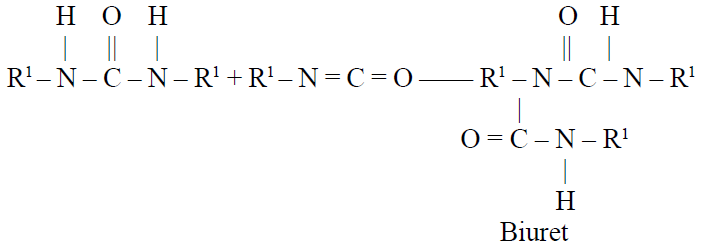
In addition to the previously mentioned components, foam formulations may contain one or a combination of additives to give specific properties to the foam products. Among these additives are those used to modify the burning characteristics of the foams: pigments; bacteriostats; inorganic fillers, such as glass fiber, silica, and barium sulfate; organic fillers, such as melamine and phosphate ester plasticizers; antistatic agents; UV stabilizers; cell openers; and internal mold release agents.





